Wisconsin Nitrate Film Project
Introduction
The complete and definitive account of the physiochemical analysis conducted by the Wisconsin Nitrate Film Project, as prepared by Professor Mahesh Mahanthappa on behalf of the chemistry group, can be found in our official white paper, available here. What we present below and on subsequent pages of this site is a layperson summary that draws from Dr. Mahanthappa’s more comprehensive account.
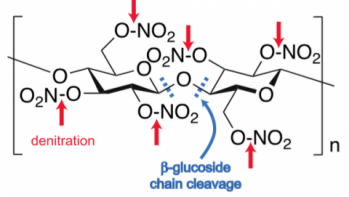 Cellulose nitrate is an intrinsically unstable material. It decomposes by way of a polymer degradation process that combines hydrolysis of its constituent nitrate esters and reactions that cleave the polymer chains into lower molecular weight species. That is, water breaks the bonds holding the nitrate groups to the cellulose backbone (shown in the red arrows in the neighboring image), in a process called denitritation. Additional reactions break apart the polymer chains that comprise the cellulose backbone (shown in the blue dashes). Cellulose nitrate’s decomposition process is autocatalytic: as it breaks down, it generates byproducts that only further the chemical decomposition reactions.
Cellulose nitrate is an intrinsically unstable material. It decomposes by way of a polymer degradation process that combines hydrolysis of its constituent nitrate esters and reactions that cleave the polymer chains into lower molecular weight species. That is, water breaks the bonds holding the nitrate groups to the cellulose backbone (shown in the red arrows in the neighboring image), in a process called denitritation. Additional reactions break apart the polymer chains that comprise the cellulose backbone (shown in the blue dashes). Cellulose nitrate’s decomposition process is autocatalytic: as it breaks down, it generates byproducts that only further the chemical decomposition reactions.
This polymer degradation process leads to noticeable changes in the physical properties of nitrate film – changes with which many archivists and conservators are quite familiar. The film becomes yellow and brittle and emits a noxious odor; it grows sticky and the emulsion softens, destroying the image; the film congeals into a solid mass; and finally, it disintegrates into a brownish powder.
The Wisconsin Nitrate Film Project employed a variety of chemical analysis and polymer characterization techniques in order to better understand the changes in nitrate film’s chemical structure and to identify how these transformations affect its physical properties, particularly, its flammability.
 These analyses were performed on samples of deaccessioned heritage nitrate film donated from the Wisconsin Center for Film and Theater Research (WCFTR) and the Wisconsin Historical Society (WHS) and on samples of “brown powder” donated from additional repositories. Our research utilized a sample set of seven rolls of motion picture film and four collections of brown powder. Although this sample set may appear limited, it is comparable to those used in previous investigations of nitrate decomposition. More information about our sample size and selection can be found on the Sample Orign & Size page of this website.
These analyses were performed on samples of deaccessioned heritage nitrate film donated from the Wisconsin Center for Film and Theater Research (WCFTR) and the Wisconsin Historical Society (WHS) and on samples of “brown powder” donated from additional repositories. Our research utilized a sample set of seven rolls of motion picture film and four collections of brown powder. Although this sample set may appear limited, it is comparable to those used in previous investigations of nitrate decomposition. More information about our sample size and selection can be found on the Sample Orign & Size page of this website.
Our physical and chemical testing focused on four separate efforts:
- Visual analyses to determine whether correlations exist between the physical appearance of heritage nitrate film and its chemical condition
- Quantitative physicochemical characterization of nitrate film samples in varying states of decay
- Accelerated aging of nitrate film samples under elevated temperature conditions at varying relative humidity levels and subsequent analyses of these samples’ chemical characteristics
- Assessments of the shock– and friction–sensitivity of four different “brown powder” samples
Each of these efforts is described in more detail on the following pages of this website, as well as in our white paper.
Results in Brief
First of all, our studies indicated that the five-stage classification model commonly employed by archives to assess the state of their nitrate holdings (described on the Sample Origin & Size page) accurately correlates with only a few physical, chemical, and flammability properties of cellulose nitrate films. While this model does have some utility for archivists and conservators, it does not always accurately reflect the true chemical state of the film or its potential flammability.
We also determined that our use of low-cost inspection tools and protocols could not determine the potential dangers of nitrate film. Correlations between visual observations and the results of our chemical analysis were poor, making it impossible to deduce flammability by observing the physical condition of the film.
We did determine, however – with the caveat that we tested a limited number of samples – that the brown powder generated in the final stage of nitrate film’s decomposition is non-hazardous. Our samples of brown powder proved insensitive to ignition by impact and friction, according a well-accepted international standard for the transport of hazardous materials.
An unexpected finding from our accelerated aging trials was that 80 % relative humidity (RH) aging conditions led to the fastest degradation of the film image, but without significantly decreasing its combustibility. The 30 %RH aging conditions led to better image preservation, yet our samples tended to depolymerize into a sticky liquid. However, the intermediate 50 %RH aging condition led to better image retention and the degradation of the film into a relatively non-hazardous byproduct.
