Wisconsin Nitrate Film Project
Introduction
A number of accelerated aging trials of nitrate film under fixed relative humidity conditions at elevated temperatures have been previously conducted by the chemical and motion picture communities. Based on the findings in these previous studies, we sought to use our advanced chemical characterization tools to gain deeper insights into how storage environments impact the degradation and flammability profiles of heritage nitrate film over an extended period of time.
We therefore set up our own 365-day accelerated aging trial for our CN-0, CN-2, and CN-4 samples at 60 °C under various relative humidity conditions (%RH). We removed our samples from these conditions at various aging time points and studied their physical and chemical properties using visual inspection, the IPI acidity test, Size Exclusion Chromotography, Proton Nuclear Magnetic Resonance Spectroscopy, and Thermogravimetric Analysis (all of these tests are described on the Physiochemical Analysis page of this website).
The major objective of this study was to understand whether or not nitrate film becomes more thermally unstable or flammable over time, as suggested by historical accounts of nitrate film fires.
The definitive account of our accelerated aging trial, as written by Professor Mahesh Mahanthappa on behalf of the chemistry group, can be found in our official white paper, which we invite you to read here. What we present below is a layperson’s summary of Dr. Mahanthappa’s more authoritative account.
Accelerated Aging Protocols
The video below, narrated by Milton Repollet-Pedrosa, one of the chemistry group project assistants, explains and visualizes the parameters and equipment used in our accelerated aging trial. Below that, you will find a prose description of our accelerated aging trial and a summary of our findings.
We focused our accelerated aging trial on our pristine CN-0 film samples, recognizing that nitrate film in more advanced stages of decay is unlikely to be kept in archival collections, particularly once the emulsion has disintigrated. However, in order to better understand how more degraded nitrate film behaves, we also included a smaller number of CN-2 and CN-4 samples in our aging trial.
The CN-0 samples were aged under 25, 50, and 80 %RH at 60 °C in separate controlled environment chambers. Our CN-2 and CN-4 samples were aged only under the most aggressive 80 %RH condition at 60 °C, also in separate environmental chambers. These conditions were selected in order to mimic storage environments ranging from a refrigerator to a humid film vault lacking careful humidity control.
 Five contiguous film frames from each sample reel were cut, stacked, and fastened together using a Teflon-coated copper wire threaded through the sprocket holes located at the edges of the film. These frame stacks were meant to mimic the conditions of rolled film stored on reels within a film canister, within the limited space of our oven.
Five contiguous film frames from each sample reel were cut, stacked, and fastened together using a Teflon-coated copper wire threaded through the sprocket holes located at the edges of the film. These frame stacks were meant to mimic the conditions of rolled film stored on reels within a film canister, within the limited space of our oven.
Each film stack was then placed in a test tube, and six film-loaded test tubes were placed in glass jars containing a seventh test tube filled with a specific saturated water/salt mixture to maintain the desired relative humidity condition at 60 °C within the container:
- a saturated MgCl2(aq) solution for 30 %RH
- a saturated NaBr(aq) solution for 50 %RH
- a saturated KCl (aq) solution for 80 %RH
The containers were then placed in an oven thermostatted at 60 ± 2 °C. Relative humidity in the containers was measured at periodic intervals using commercially-available NIST traceable hygrometers.
By removing sample stacks after 15, 30, 60, 90, 180, and 360 days of accelerating aging, we assessed how the physical and chemical state of the film changed as a function of time. Standard chemical reaction rate theories suggest that the rate of decomposition for heritage nitrate film approximately doubles with every ~10 °C increase in the reaction temperature. Assuming that the degradation of our nitrate samples follows this established rate:
- the 90-day time point in our 60 °C accelerated aging tests corresponds to ~13.8 years of aging in a 36 °F (2.2 °C) refrigerator
- 365 days of accelerated aging at 60 °C corresponds to ~55.7 years of storage in a 36 °F refrigerator or ~220 years in a 0 °F (-18 °C) freezer
Accelerated Aging of CN-0 at 30%RH
Aging CN-0 at 60 °C under a 30 %RH atmosphere caused significant changes in the physical appearance of the film samples, as can be seen in the images below.
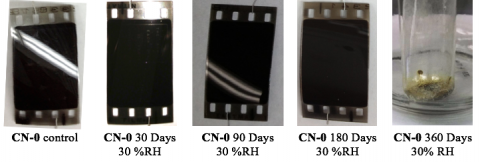
The physical appearance of the film was relatively unchanged after up to 90 days, and only a small amount of a brown gas was observed to build up in the sealed aging chamber over time. We tentitavely identified this as NO2(g), a byproduct of the thermal decomposition of nitric acid (HNO3) liberated from the film by hydrolysis.
After 180 days of accelerated aging, the samples lost their sheen. Remarkably, the film degraded into a puddle of viscous, pale yellow liquid after ~240 days of aging. We tentatively concluded this was an aqueous solution of partially nitrated glucose oligomers, and it may be related to the “viscous froth” that appears on the surface of degraded nitrate film that falls under the Stage 4 classification.
Detailed analyses of the chemical constitution of the film revealed that this 30 %RH aging condition does not appear to cause the denitration of the film and suggest that the film remains largely intact until catastrophic degradation occurs at longer times. Nuclear Magnetic Resonance (NMR) analysis of the pale yellow liquid resulting from catastrophic decomposition reveals complete degradation of the polymer backbone to yield nitroglucose derivatives, with no signs of intact cellulose nitrate polymer.
Accelerated Aging of CN-0 at 50%RH
CN-0 aged under a 50%RH atmosphere at 60 °C displayed a different course of physical degradation, as shown in the images below.
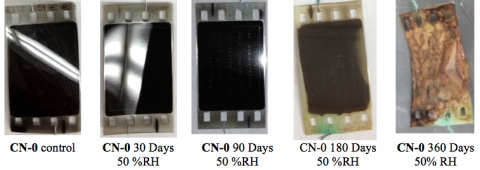
In the first 90 days of the film aging, the samples appeared physically unchanged but a significant amount of a brown gas (again surmised to be NO2(g)) was observed to accumulate in the sealed chamber.
After 180 days, the film lost its sheen and the emulsion became uneven and sticky.
At the final 360 day time point, we found that the film samples had become extremely brittle, rusty brown solids. These final samples exhibited many of the physical attributes of deterioriated film stock that may ultimately decay into brown powder.
Our chemical analysis showed evidence of decay by denitration. We also observed that film aged for 360 days at 50%RH is not combustible.
Accelerated Aging of CN-0 at 80%RH
Aging CN-0 at 60 °C under an 80 %RH atmosphere resulted in the fastest degradation of the emulsion and yellowing of the nitrate base, as evident in the images below.
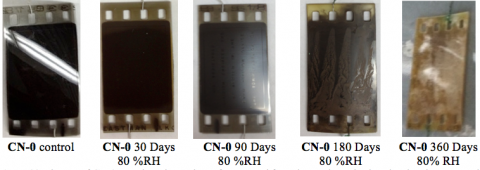
After only 90 days under these conditions, the film emulsion was sticky and the base had yellowed. These changes in the physical appearance of the film were accompanied by the formation of brown NO2(g) in the aging chamber. The gaseous product seemed to react further with the gelatin layer. Thus, our pristine Stage 0 film had degraded to a Stage 2 after only 90 days in our aging chamber.
Degradation proceeded further to yield a liquid-like emulsion layer and a brown film base after 180 days of aging.
Complete image degradation and embrittlement of the yellow-brown nitrate base layer occured after 360 days.
Our subsequent analysis showed that the chemical composition of the film did not appear to appreciably change during aging at 80 %RH. These chemical findings are strikingly discordant with the results of the visual inspection of these aged samples at each time point, providing further evidence that the five-stage classification model for nitrate film degradation does not always accurately reflect the true chemical state of the film or its potential hazards.
We concluded that the high relative humidity condition is the worst for image preservation and that the resulting product of decomposition retains its high flammability.
Accelerated Aging of CN-2
In order to better understand how a partially degraded film sample decomposes, we aged samples of CN-2 under 80 %RH conditions for up to 360 days. We selected this aging condition by virtue of our initial intuition that higher relative humidity conditions would lead to greater levels of denitration and dramatic changes in the flammability profile of the film.
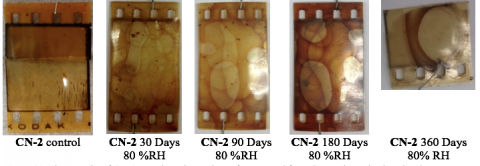
As you can see in the images above, our sample degraded from Stage 2 to Stage 3 after 30 days, as evidenced by the formation of bubbles in the emulsion layer. This change was accompanied by increased acidification. However, we noted that the acidity of the film recovered to some extent at longer times, possibly due to the equilibrium formation of gaseous nitrogen oxides. Since our sampling methodology involved opening the sealed container to extract samples at prescribed time points, the nitrogen oxides were allowed to escape from the container. By effectively removing the nitrogen oxides from the aging canister atmosphere, we may have artificially decreased the detected acidity of the film.
Our sample set showed variability in its combustibility, the orgins of which remain obscure.
Accelerated Aging of CN-4
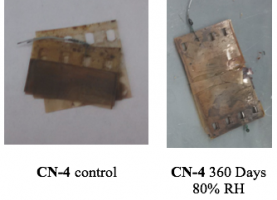 As a corollary study to our CN-2 aging trials, we also aged one sample of CN-4 for 360 days at 80 %RH. We limited this analysis to a single data point after 360 days of accelerated aging, due to space limitations and the fact that films at this advanced stage of decay lack any intrinsic value due to the complete degradation of the image. This analysis thus aimed only to understand the final stages of nitrate film decompostion.
As a corollary study to our CN-2 aging trials, we also aged one sample of CN-4 for 360 days at 80 %RH. We limited this analysis to a single data point after 360 days of accelerated aging, due to space limitations and the fact that films at this advanced stage of decay lack any intrinsic value due to the complete degradation of the image. This analysis thus aimed only to understand the final stages of nitrate film decompostion.
As with the aged CN-2 samples, aging CN-4 for 60 days at 80 %RH resulted in the film becoming more brittle and discolored, as evident in this image.
Our chemical analysis showed evidence of some denitration; however, the level of denitration was low. The film thus retained its flammability under the high relative humidity aging condition long after the image was degraded beyond use.
Cumulative Analysis of Acclerated Aging Data
Comparison of the data collected on all of the film samples subjected to accelerated aging trials provides some new insights into the deterioration behavior of cellulose nitrate film.
Our results were somewhat surprising, as we initially expected that the denitration process would occur most quickly for the samples aged at the highest relative humidity condition (80 %RH). This expectation was based on the notion that water is required for the hydrolysis of the nitrate esters – the cleaving of the nitrate groups from the cellulose backbone. That is, higher water concentrations should drive denitration.
However, the data suggest that the 50 %RH condition drives denitration to the greatest extent, and that the 30 %RH condition results in hydrolysis of both the nitrate esters and the backbone beta-glucoside linkages to yield a syrupy solution. Data further demonstrate that the molecular weight of the film base is relatively unchanged at the highest relative humidity condition, yet the molecular weight drops upon aging at lower relative humidities.
In short, we observed high levels of film denitration at lower relative humidity aging conditions. This is because the rate of the hydrolysis reaction depends on the concentrations of several reactants – specifically, nitrate esters, nitric acid (HNO3), and water.
At low relative humidity, with less water present, the effective active concentration of nitric acid is also relatively low because it is less hydrated, and so it is a less effective catalyst of the hydrolysis reaction. When more water is present at intermediate relative humdities, the effective active conentration of nitric acid increases and forms H3O+, which catalyzes nitrate ester hydrolysis. At very high relative humidities, the H3O+ ions become so stable that they are unavailable to participate in the hydrolysis reaction. Therefore, hydrolysis is fastest at intermediate water concentrations and intermediate relative humidities.
In other words, the rate of the hydrolysis reaction is expected to peak at some intermediate relative humidity. We found that the 50 %RH humidity accelerated aging condition initially enabled maintenance of the film image prior to decomposition into a non-hazardous solid.
