Wisconsin Nitrate Film Projecty
Our initial stage of physiochemical analysis sought to assess whether there are correlations between the five-stage model of decomposition commonly employed by film archives, the molecular composition of nitrate film, and the film’s ultimate thermal stability. A substantial portion of existing literature claims that the combustibility of nitrate films increases in the later stages of decomposition, but a few exceptional references suggest that flammability actually decreases in advanced states of decay.
To assess the validity of these conflicting claims, we employed six different tools in the study of our three heritage nitrate film samples:
- the Image Permanence Institute (IPI) Acidity Test for nitrate film
- size-exclusion chromatography (SEC) to directly measure the molecular weight of the cellulose nitrate base and to correlate it to the attributed stage of decay
- combustion elemental analysis to determine the composition of the film base
- proton nuclear magnetic resonance (1H NMR) spectroscopy of dissolved nitrate film bases to understand their chemical structures, especially, to quantify the nitration level of each sample
- thermogravimetric analysis (TGA) to assess the temperatures at which these samples begin to decompose or deflagrate
- differential water sorption studies to measure water uptake of nitrate film samples
The definitive account of these analyses and their results, as written by Professor Mahesh Mahanthappa on behalf of the chemistry group, can be found in our official white paper, which we invite you to read here. Full descriptions of the experimental procedures, analytical instruments, and analysis parameters used can be found here.
What we present below is a layperson summary of Dr. Mahanthappa’s more authoritative account. We have attempted to describe these analyses and their results in brief, and have illustrated several of our key tests with short explanatory videos.
As explained in more detail below and in our white paper, this first stage of physiochemical analysis indicated that the five-stage model of nitrate film decomposition accurately correlates with only a few physical and chemical properties of cellulose nitrate film.
- Stages of decay do correlate with acidity, nitration levels, and decomposition onset temperatures.
- Stages of decay do not correlate with the molecular weight of the film base, the amount of campor present, water sorption characteristics, or deflagration temperature
The IPI Acidity Test
 Based on previous observations that nitrate film stock becomes increasingly more acidic as it decays by virtue of denitration and other chemical processes, the Image Permanence Institute (IPI) established a standardized test for nitrate film known as the IPI Acidity Test.
Based on previous observations that nitrate film stock becomes increasingly more acidic as it decays by virtue of denitration and other chemical processes, the Image Permanence Institute (IPI) established a standardized test for nitrate film known as the IPI Acidity Test.
We adopted a variant of this test, in which we soaked CN-0, CN-2, and CN-4 samples in deionized water to leach any acids into the aqueous solution. We then measured the pH of the solution using pH paper.
Our results were consistent with the general notion that pristine nitrate film exhibits a detectable acidity, and that the material becomes more acidic as it decays.
Size Exclusion Chromatography (SEC)
One common notion about the decomposition of nitrate film is that the level of decay is correlated with the molecular weight of the film base. This conventional wisdom is because one known method of degradation is by an acid-catalyzed cleaving of the cellulose nitrate backbone, which would decrease the mechanical stability of the film and lead to the embrittlement typical of Stages 4 and 5 in the five-stage model of decomposition. Stimulated by this idea, we sought to quantify the molecular weights and molecular weight distributions of the film base using a well-known methodology in polymer science: size exclusion chromatography (SEC).
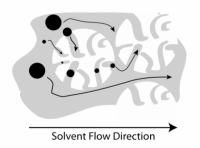 SEC relies on a separation of polymer chains based on their size upon dissolution in a solvent. The polymer solution passes through columns of gel beads that have a variety of pore sizes; the beads sieve the polymers by size. Polymers that are too large to enter the gel beads pass through the column first, while smaller polymers (polymers with lower molecular weight), are slowed down by their passage through the gel. By monitoring the polymer concentration in solution exiting the columns over time, and comparing the elution time to a reference standard, one can measure the molecular weight and molecular weight distribution of the polymer being tested.
SEC relies on a separation of polymer chains based on their size upon dissolution in a solvent. The polymer solution passes through columns of gel beads that have a variety of pore sizes; the beads sieve the polymers by size. Polymers that are too large to enter the gel beads pass through the column first, while smaller polymers (polymers with lower molecular weight), are slowed down by their passage through the gel. By monitoring the polymer concentration in solution exiting the columns over time, and comparing the elution time to a reference standard, one can measure the molecular weight and molecular weight distribution of the polymer being tested.
For our study, our film samples were treated with bleach (5 wt% NaOCl) to remove the emulsion layer, which enabled us to focus only the cellulose nitrate base. The bleached film was then dissolved and eluted through two commercial analytical SEC columns. The video below, featuring Milton Repollet-Pedrosa, one of the chemistry group project assistants, briefly demonstrates how our samples were prepared.
The results of our initial SEC analyses allowed us to draw comparisons between our three heritage film samples, CN-0, CN-2, and CN-4. These results indicated that the molecular weight of the film base is not at all correlated with the age of the film nor its level of decay. Therefore, we conclude that film molecular weight is not related to the five-stage condition classification.
We speculate that variable raw materials and specific manufacturing and processing conditions affected the molecular weight of the nitrate base of our film samples. Most nitrate film stocks were manufactured prior to the widespread acceptance of the existence of high molecular weight polymers by the scientific community, and so analytical methods for controlling the molecular weight of the film base likely did not exist. As a point of reference, analytical SEC was only invented and first disclosed in 1955 – four years after the discontinuation of nitrate film manufacture.
Quantitative Elemental Analysis
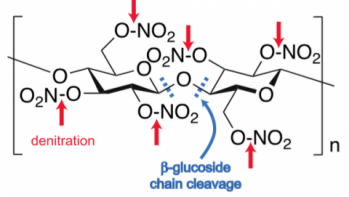 The degradation of cellulose nitrate occurs by two hydrolysis (literally, “cutting by water”) mechanisms:
The degradation of cellulose nitrate occurs by two hydrolysis (literally, “cutting by water”) mechanisms:
- cleavage of the nitrate groups from the cellulose backbone in a process called “denitration,” (indicated by the red arrows in the neighboring diagram)
- cleavage of the beta-glucoside linkages that comprise the cellulose backbone (indicated by the blue dashes)
Denitration is most likely an autocatalytic process, since it liberates nitric acid (HNO3), which can further catalyze both denitration and beta-glucoside cleavage of the polymer chains. Moreover, both processes reduce the suppleness and overall mechanical integrity of the material in a manner consistent with advanced stages of nitrate film degradation.
We hypothesized that the five-stage model of nitrate film deterioration would correlate strongly with the level of film denitration. Not only would the loss of the nitrogen groups create hydrogen bonds that increase the rigidity of the base, but the nitric acid liberated by this process would also degrade the emulsion layer by breaking down the gelatin and oxidizing the silver particles.
To test this hypothesis, we determined the elemental composition of our samples using quantitative analysis of the gases generated during controlled combustion. Sample CN-0, CN-2, and CN-4 frames were bleached to remove the emulsion layer, allowing us to focus only on the cellulose nitrate base. The samples were sent to Columbia Analytical Labs (Tuscon, AZ) to determine their carbon, hydrogen, nitrogen, and sulfur (CHNS) contents.
We included sulfur in our analysis because cellulose nitrate manufacturing typically employed sulfuric acid (H2SO4) as a catalyst. However, if sulfuric acid becomes covalently attached to the polymer backbone, it could impact the long-term stability of the film by also catalyzing the denitration process.
We expected to observe lower nitrogen content in the more severely degraded samples, and the results were consistent with this hypothesis. The change in nitrogen content was small, but its impact on the brittleness of the film sample was readily apparent: CN-4 is much more brittle than the supple CN-0. However, the nitrogen content was also lower than expected for the excellent condition of CN-0, which we believe is related to the presence of camphor, as discussed in the following section.
Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
Solution proton nuclear magnetic resonance (1H NMR) spectroscopy is a common chemical analysis tool for determining the structure and atomic connectivity of organic molecules that contain both carbon and hydrogen. When placed in a magnetic field, carbon and hydrogen nuclei absorb electromagnetic radiation at particular frequencies. Those frequencies are dictated by the surrounding chemical environment – that is, by what other atoms are linked to the hydrogen or carbon. By measuring the frequencies at which nuclei absorb electromagnetic radiation, 1H NMR indicates what types of chemical bonds or function groups are present.
We performed 1H NMR analysis on bleached CN-0, CN-2, and CN-4 samples that were dissolved in a solvent, called DMSO-d6. By removing the gelatin from the samples with bleach (5 wt% NaOCl), we were able to focus solely on the structure of the cellulose nitrate film base.
The video below, featuring Milton Repollet-Pedrosa, one of the chemistry group project assistants, briefly demonstrates how our samples were prepared for 1H NMR analysis.
The resulting 1H NMR spectra allowed us to identify various nitration patterns along the cellulose backbone, which in turn enabled calculation of the degree of nitration in each sample. Nitration level again correlated with stage of decay, as the samples in more advanced stages of decomposition were less heavily nitrated. Though the difference in degree of nitration was small, it may account for the decreased flexibility of the CN-2 and CN-4 samples. It is also possible, however, that the relative brittleness of these samples is caused by different phenomena.
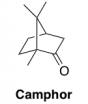 Significantly, analysis of our 1H NMR spectra also revealed a significant quantity of camphor. Camphor was used as a plasticizer in the manufacture of nitrate film to increase the suppleness of the base. We found that camphor accounted for as much as 20 wt% of the samples, which explains the lower than expected nitrate content that we observed in our elemental analysis (described above).
Significantly, analysis of our 1H NMR spectra also revealed a significant quantity of camphor. Camphor was used as a plasticizer in the manufacture of nitrate film to increase the suppleness of the base. We found that camphor accounted for as much as 20 wt% of the samples, which explains the lower than expected nitrate content that we observed in our elemental analysis (described above).
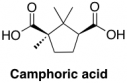 Nitric acid (HNO3), released during the denitration process, reacts with camphor to produce camphoric acid, which in turn reduces the amount of effective plasticizer in the sample. An increased amount of camphoric acid may thus contribute to the increased brittleness of more degraded nitrate film.
Nitric acid (HNO3), released during the denitration process, reacts with camphor to produce camphoric acid, which in turn reduces the amount of effective plasticizer in the sample. An increased amount of camphoric acid may thus contribute to the increased brittleness of more degraded nitrate film.
To the best of our knowledge, this degradative reaction of the plasticizer and its impact on nitrate film stability has not been previously documented in nitrate literature.
The presence of camphor in these films is also significant for fire protection purposes, as camphor is highly volatile and combustible. While we do not know the initial camphor content in the film at the time of manufacture, the high amount of it in our samples suggests that it plays a role in nitrate’s flammability.
We thought it was somewhat surprising that camphor’s distinctive odor is not dectable when handling nitrate film. Upon abrading the surface of a single CN-0 frame with sand paper, we could smell the camphor. Since abrasion apparently affects camphor’s ability to escape from the film, we attempted to quantify the level of surface abrasion of the film samples with their physical properties as part of our visual inspection project (described on the Visual Inspection & Analysis page of this website). However, we were unable to correlate surface abrasion with the overall condition of the film, and so we did not continue this line of investigation.
Thermogravimetric Analysis (TGA)
 Thermogravimetric analysis (TGA) is a well-known method for studying the thermal stability of polymer samples. In this technique, a small sample of the polymer is placed on a sensitive balance inside a chamber where the temperature is increased at a constant rate over time, under a flow of oxygen. The scale measures the changing mass of the sample and can thus determine the temperature at which the polymer fully decomposes.
Thermogravimetric analysis (TGA) is a well-known method for studying the thermal stability of polymer samples. In this technique, a small sample of the polymer is placed on a sensitive balance inside a chamber where the temperature is increased at a constant rate over time, under a flow of oxygen. The scale measures the changing mass of the sample and can thus determine the temperature at which the polymer fully decomposes.
Admittedly, TGA does not directly mimic the decomposition of nitrate film in a canister within a film vault. While TGA is a dynamic test, with pure oxygen flowing over the sample while heat is applied, a film vault is typically held at a constant temperature with modest air circulation, and any gases released by the film remain confined in the storage can with the film. To specifically understand nitrate combustibility under the conditions in an archive, one would ideally conduct time-dependent TGA at a fixed temperature of interest (e.g., 30 or 40 °C), which is a time and resource intensive undertaking. Our TGA studies, however, allowed us to glean useful information regarding the presence of any volatile compounds in our samples, while also measuring their relative flammabilities.
For our nitrate film samples, we wanted to determine the onset of decomposition – or the temperature at which 5% mass loss was observed – and the deflagration temperature – or the temperature at which the sample combusted. We took samples from the center of CN-0, CN-2, and CN-4 frames, including both the base and the emulsion layers.
This short video, narrated by Milton Repollet-Pedrosa, one of the chemistry group project assistants, explains and demonstrates the TGA process, and also features a controlled burning of a nitrate film sample in a glass vessel that attempts to simulate what happens to the film inside the TGA chamber.
The TGA profiles of our samples indicated that the decomposition onset temperature decreased with increasing stages of decay; that is, our CN-4 samples began to decompose at lower temperatures than our CN-0 samples (note that the reasons for this mass loss are not known). The deflagration temperatures of all the samples were reasonably similar. Spontaneous combustion of the samples typically left behind a residue that decomposed at temperatures above 300 °C.
 We also found that the bubbling of the emulsion caused an apparent initial increase in mass, followed by combustion with significant mass loss. In order to understand whether the emulsion layer affects the combustibility of the film, we tested additional samples with the emulsion layer removed by bleach. Subsequent TGA analyses of the bleached sample exhibited indistinguishable profiles, suggesting that the emulsion layer plays a minor role in the ultimate thermal stability of nitrate film.
We also found that the bubbling of the emulsion caused an apparent initial increase in mass, followed by combustion with significant mass loss. In order to understand whether the emulsion layer affects the combustibility of the film, we tested additional samples with the emulsion layer removed by bleach. Subsequent TGA analyses of the bleached sample exhibited indistinguishable profiles, suggesting that the emulsion layer plays a minor role in the ultimate thermal stability of nitrate film.
Differential Vapor Sorption
Since water is a key ingredient in the processes that degrade cellulose nitrate film, we sought to study the water uptake characteristics of our film samples. To do so, we collaborated with Professor M. A. Hickner (Department of Materials Science Engineering at the Pennsylvania State University) to perform differential vapor sorption analyses of CN-0, CN-2, and CN-4 film samples. We tested our samples with the emulsion intact, but we also tested CN-0 samples that had the emulsion layer removed by bleach.
In these experiments, film samples were placed on a sensitive balance and equilibrated in a controlled environment at a given temperature but with varying relative humidities (%RH). We tested our samples at both 30 °C and 60 °C and at a wide variety of %RH conditions. By measuring the equilibrium mass of the samples under each %RH condition, we could calculate the percentage of water uptake and plot a differential vapor sorption curve.
We found little difference in the water uptake in our film samples with the emulsion intact, suggesting that water uptake is nearly independent of the condition of the film image. That is, our pristine CN-0 samples absorbed approximately the same amount of water as our highly degraded CN-4 samples. However, our bleached CN-0 sample showed a sharp decrease in water uptake. Unfortunately, this suggests that the emulsion – the layer holding the image that we wish to preserve – acts as sponge that draws water to the film that accelerates its degradation.
